
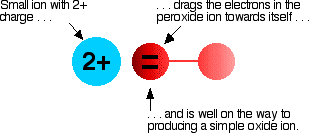

Your answer should include: Diatomic / Poisonous / Yellow Describe the look and nature of Chlorine at room temperature. If it is the more reactive molecule that is in the compound, no reaction will occur.ĭescribe the look and nature of Fluorine at room temperature. For example, fluorine will displace chlorine in hydrogen chloride to produce hydrogen fluoride. Displacement Reactionsīe able to work out if a displacement reaction will occur between a halogen compound and a different halogen molecule.īe able to write the equation for any displacement reactions that may occur between halogen compounds and halogen molecules.Ī more reactive halogen will replace another in a compound. The halogen atom takes an electron from metal atom. Halogens form ionic bonds with other metal _atoms _when they react. Halogens form covalent bonds with other non-metal atoms when they react. As you move down the group, the amount of electron shielding increases, meaning that the electron is less attracted to the nucleus. What happened to reactivity down the group 17 The chemical reactivity of group 17 elements decreases down the group. This is because group 7 elements react by gaining an electron. This is because group 7 elements react by gaining an electron. Relative atomic mass increases down the group. The evidence that support my statement is Table B from. This is because the atomic radii increase down the group, increasing the amount of intermolecular forces holding each molecule together. The trend is that from top to bottom in the alkali metals group, the reactivity levels increase. Melting/boiling points increase down the group. For this reason, fluorine is the most reactive halogen and astatine is the least reactive of the halogens. This is because group 7 elements react by gaining an electron. How relative atomic mass increase down group 7 and why. How melting and boiling points increase down group 7 and why. How group 7 elements react with metals and nonmetals. Group 1Reactions with Metals and Non Metals Iodine is a poisonous grey solid or a purple gas.This is because the further away an electron is from the nucleus, the weaker its attraction and the more likely it is to react with another atom. The reactivity increases down the group from Mg to Ba. The first electron to react will be on the outer shell.

Bromine is a poisonous brown liquid or orange gas. So as you go down the group there are more energy levels, increasing the atomic radius.The increased distance and the increased shielding weaken the nuclear attraction, and so an atom can’t attract electrons as strongly. They are coloured vapours at room temperature: Down a group, the number of energy levels (n) increases, and so does the distance between the nucleus and the outermost orbital. They are diatomic (travel in pairs, i.e Br2) This can happen easiest if the electron is in a shell that is a long ay from the nucleus so that there is less attraction between the nucleus and the electron. Group 7 elements are known as the halogens. the attraction between the nucleus and outer electron gets weaker as you go down the group so the electron is more easily lost.

Why does reactivity decrease down a group The reactivity of Group 7 elements decreases down the group. the force of attraction between the nucleus and the outer electron decreases. the outer electron becomes further from the nucleus.


 0 kommentar(er)
0 kommentar(er)
